SINT1A
Supplementation with B. infantis to mitigate Type 1 Diabetes Autoimmunity
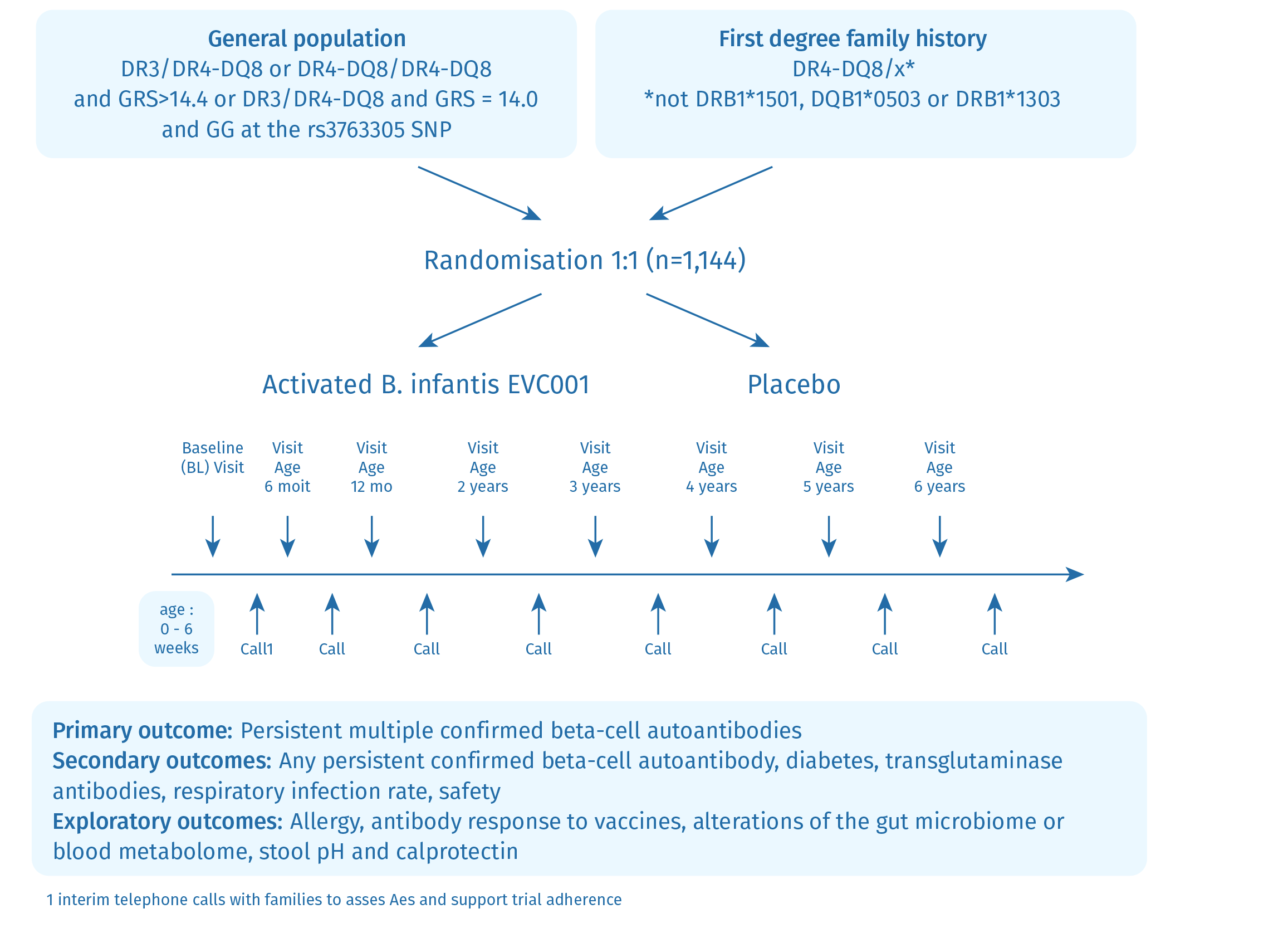
The SINT1A study is a randomized, placebo-controlled, double-blind, primary prevention study designed to demonstrate the potential benefits of Bifidobacterium infantis in preventing islet autoimmunity and type 1 diabetes in genetically high-risk children. The intervention involves the daily administration of the probiotic or a placebo given to infants from the age of 7 days to 6 weeks until they reach 12 months. The SINT1A study is conducted in five countries: Germany, UK, Poland, Belgium and Sweden.
Enrollment Completed
As of early 2024, enrollment has been completed with a total of 1149 infants participating in the study. In addition to the primary focus on islet autoimmunity and type 1 diabetes, the study also examines other health outcomes such as celiac autoimmunity, respiratory infections, allergy, antibody response to vaccines, and alterations in the gut microbiome. This broad approach allows for a more comprehensive understanding of the probiotic’s effects on various aspects of health.
Information for Participants
Is your child currently participating in one of our studies? Are you interested in joining our new prevention study AVAnT1A? Or are you seeking more information regarding the genetic risk for type 1 diabetes of your child?

