POInT
Primary Oral Insulin Trial
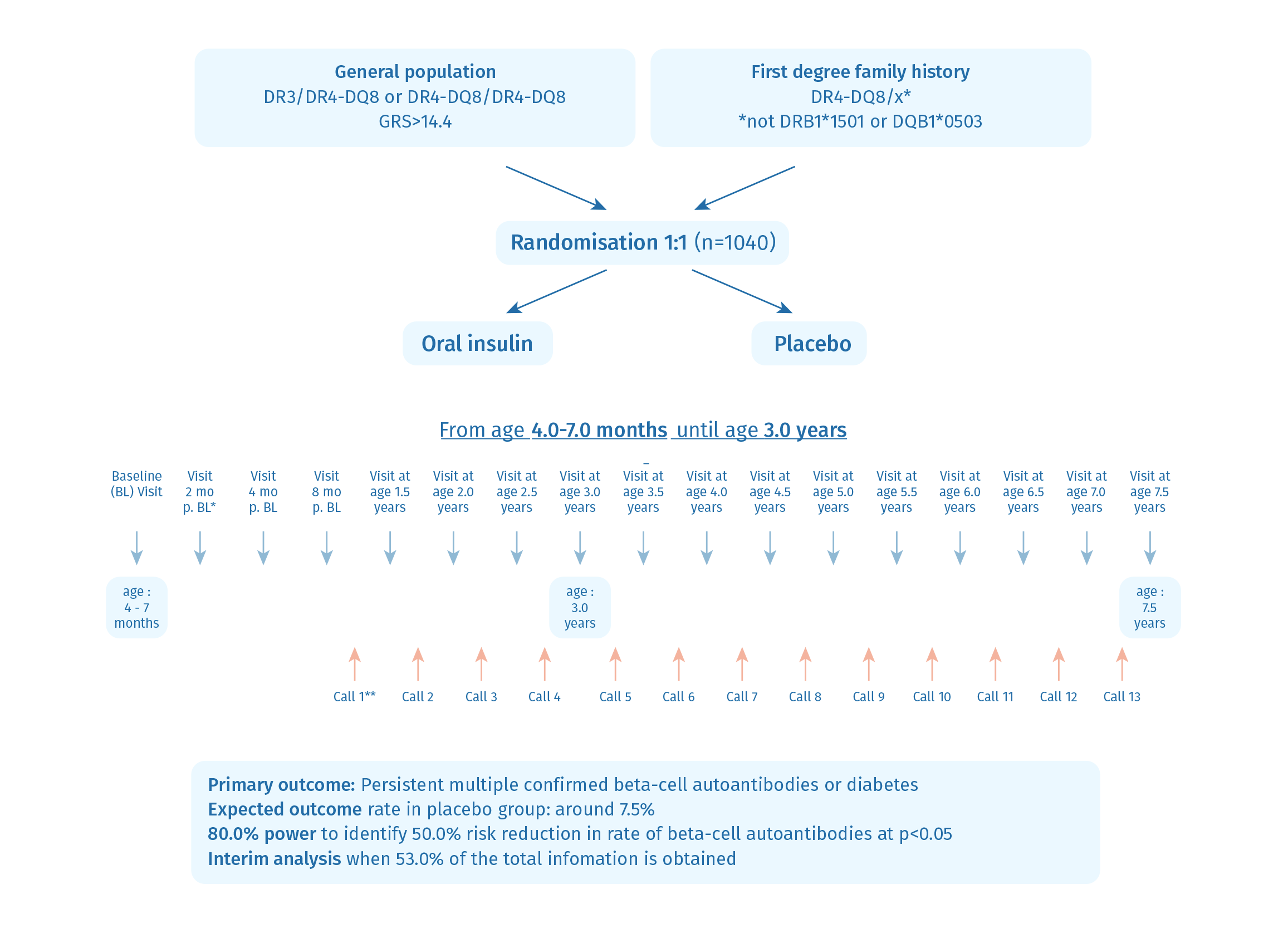
The POInT study is a randomized, placebo-controlled, double-blind primary prevention clinical trial designed to investigate the hypothesis that repeated exposure to oral insulin during the critical period when islet autoimmunity typically begins will induce immune tolerance to islet autoantigens and, thereby, decrease the likelihood of islet autoimmunity and the onset of type 1 diabetes in childhood. This rationale is based on findings suggesting that the loss of tolerance against insulin is a pivotal event in the development of type 1 diabetes and the demonstration that oral tolerance can be achieved in chronic inflammatory conditions.
Study Design
The trial was performed in children with an elevated genetic risk for type 1 diabetes. Participants received either oral insulin with a dose escalation from 7.5 mg to 22.5 mg to 67.5 mg, or a placebo daily starting from 4 months to 7 months of age until the child’s 3rd birthday. The primary objective is to reduce the cumulative incidence of islet autoantibodies and diabetes in childhood. The trial commenced in February 2018 across seven clinical sites in Belgium, Germany, Poland, Sweden, and the UK, with enrollment of 1050 children now complete. The last visit was in June 2024.
Sharing Biospecimen and Data to drive Innovation
POInT also presents a unique opportunity to address crucial questions regarding the pathogenesis of type 1 diabetes and the mechanisms of antigen-based therapy. Ancillary studies have explored glucose metabolism in infancy, the psychological impact of genetic screening, the relationship between COVID-19 infections and islet autoimmunity, and the effects of growth and weight development during the pandemic. In addition to biosamples collected for trial purposes, a GPPAD biobank has been established, offering exceptional access to serum, plasma, RNA, viable PBMC, DNA, or stool samples collected during the clinical trial. To foster collaborations, GPPAD recently announced funding for five research partners in France, the USA, and the UK to access and investigate biosamples from POInT. This initiative will significantly facilitate the study of factors influencing type 1 diabetes pathogenesis in early life and the development of new therapeutic approaches to prevent or delay disease onset.
Ancillary Studies using POInT Biospecimen
In 2023, GPPAD announced a Request For Proposal (RFP) for investigators to perform innovative research on biosamples from the GPPAD POInT clinical trial utilizing cutting-edge analysis techniques relevant to pancreatic, metabolic, or immune research. The projects selected for funding complement GPPAD’s ongoing research approaches and analyses.
Information for Participants
Is your child currently participating in one of our studies? Are you interested in joining our new prevention study AVAnT1A? Or are you seeking more information regarding the genetic risk for type 1 diabetes of your child?

